Cymbalta Discontinuation Syndrome Treatment
Cymbalta discontinuation syndrome treatment. Do not start CYMBALTA in a patient who is being treated with linezolid or intravenous methylene blue because there is an increased risk of serotonin syndrome. To avoid such distress medications like duloxetine should be tapered very gradually. Drug present in human milk in a published study lactating women who were weaning their infants were given duloxetine.
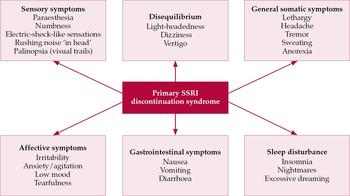
The likelihood of developing discontinuation syndrome varies by individuals the treatment and dosage prescribed said Thomas Biegi a spokesman for Pfizer maker of antidepressants like. Discontinuation syndrome As you have noted it can be extremely challenging. Increase risk of falls especially in elderly.
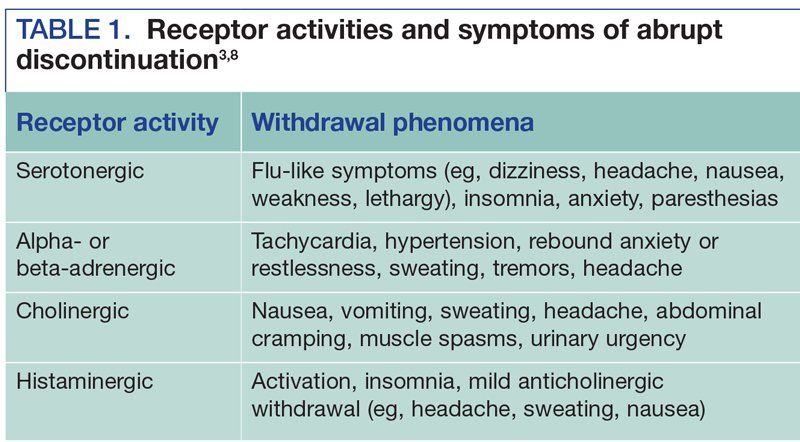
This condition can be life-threatening and symptoms may. Cymbalta Discontinuation Syndrome Severe Withdrawal Symptoms. These features are consistent with either a direct toxic effect of the SNRIs or SSRIs or possibly a drug discontinuation syndrome.
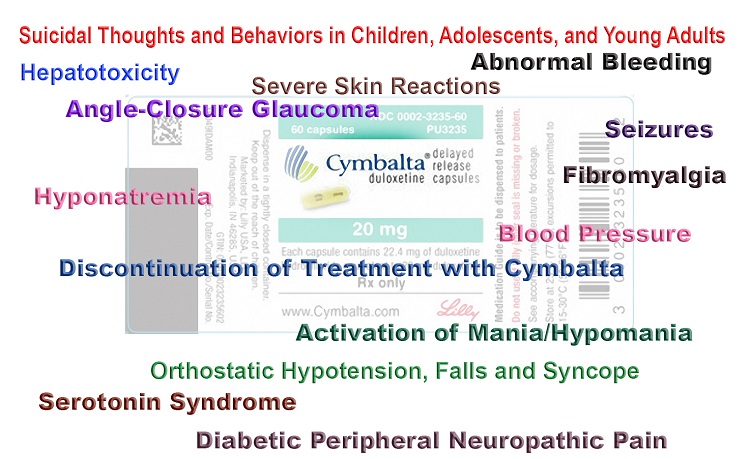
Especially when combined with other medications that increase serotonin Cymbalta may cause a life-threatening condition that includes anxiety high blood pressure rapid heartbeat rapid breathing sweating and tremors. Antidepressant discontinuation syndrome also called antidepressant withdrawal syndrome is a condition that can occur following the interruption reduction or discontinuation of antidepressant medication following its continuous use of at least a month. Selective serotonin reuptake inhibitors SSRIs and serotonin-norepinephrine reuptake inhibitors SNRIs.
In some cases the clinical picture is consistent with serotonin syndrome. In addition duloxetine is used to help relieve nerve pain peripheral neuropathy in people with diabetes or ongoing pain due to medical conditions such as arthritis chronic back pain or fibromyalgia a condition that causes widespread pain. Decrease your blood pressure when standing and cause dizziness or fainting mostly when first starting Cymbalta or when increasing the dose.
A 2005 study on generic duloxetine found that 44 of people experienced withdrawal symptoms. Withdrawal symptoms from duloxetine are so common and severe that Cymbalta Discontinuation Syndrome is a well-documented issue. Discontinuation of Treatment.
In early reports it was referred to as a withdrawal. Duloxetine Cymbalta is prescribed for pain as well as depression.
Increase your blood pressure.
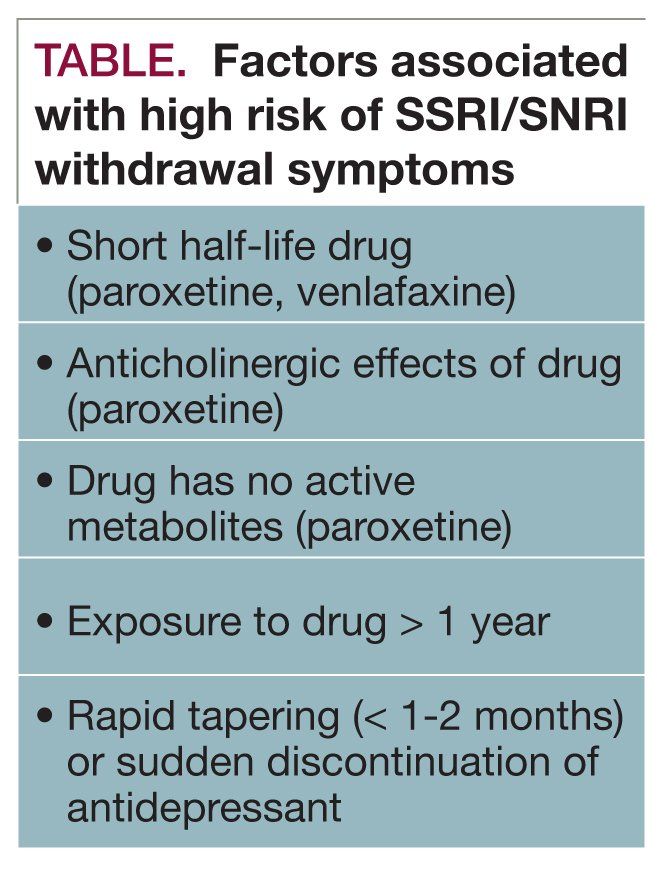
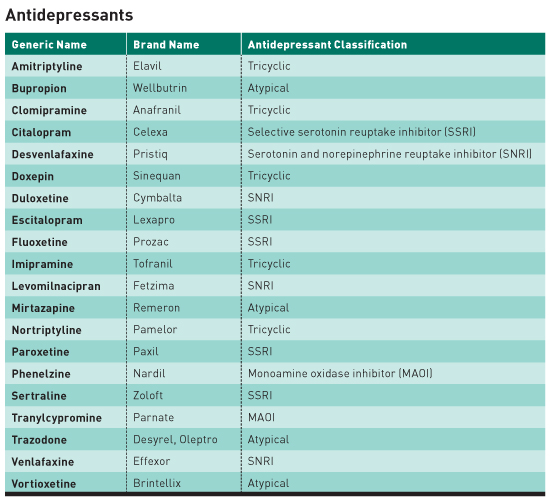
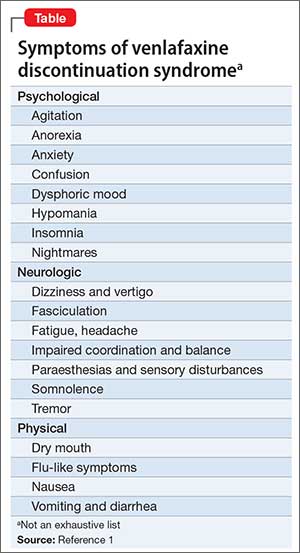
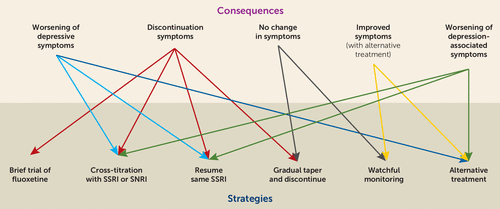
Interruption of treatment with an anti-depressant medication is sometimes associated with an antidepressant discontinuation syndrome. In doctorspeak that means it is a serotonin-norepinehprine reuptake inhibitor and is somewhat similar to other antidepressants such as Effexor venlafaxine and Pristiq desvenlafaxine. In clinical trials adverse events seen on abrupt treatment discontinuation occurred in approximately 45 of patients treated with Cymbalta and 23 of patients taking placebo. Decrease your blood pressure when standing and cause dizziness or fainting mostly when first starting Cymbalta or when increasing the dose. Cymbalta may increase the risk of bleeding events. Antidepressant discontinuation syndrome also called antidepressant withdrawal syndrome is a condition that can occur following the interruption reduction or discontinuation of antidepressant medication following its continuous use of at least a month. Cymbalta was originally approved by the FDA in 2004 for the treatment of major depression. Discontinuation syndrome As you have noted it can be extremely challenging. When discontinuing treatment with duloxetine the manufacturer recommends a gradual reduction in the dose rather than abrupt cessation whenever possible.
Duloxetine Cymbalta is prescribed for pain as well as depression. Especially when combined with other medications that increase serotonin Cymbalta may cause a life-threatening condition that includes anxiety high blood pressure rapid heartbeat rapid breathing sweating and tremors. Suicidal thoughts and behaviors. Withdrawal symptoms from duloxetine are so common and severe that Cymbalta Discontinuation Syndrome is a well-documented issue. When discontinuing treatment with duloxetine the manufacturer recommends a gradual reduction in the dose rather than abrupt cessation whenever possible. Withdrawal symptoms when treatment is discontinued are common particularly if discontinuation is abrupt see section 48. Cymbalta had a black box warning related to this concern added in 2014.
/cymbalta-withdrawal-symptoms-timeline-and-treatment-4707711_final-0cedae29ad7f4a86bb8159be527b6774.png)
:max_bytes(150000):strip_icc()/prozac-withdrawal-symptoms-timeline-and-treatment-4766892_final_edit-ddf616fc766d4651afa6b5d804721eea.png)

:max_bytes(150000):strip_icc()/Anti-depressant-withdrawal-4172110-V1-3baee0923fb14b448bb194ea4083efc7.gif)
























/tips-to-reduce-antidepressant-withdrawal-symptoms-1066835-v4-eb4906b83ce947b0ab393a20af2950f1.png)







/GettyImages-981113982-8bf1a5f4abab4ba5b8161c3e3521a865.jpg)
Post a Comment for "Cymbalta Discontinuation Syndrome Treatment"